
A look at why you need to determine whether twins are monochorionic or dichorionic.

More than half of postmenopausal women report low sexual desire. Hormonal and nonhormonal treatments vary in efficacy and continued studies are needed.

US obstetricians need to once again be concerned about maternal iodine deficiency.

Sobering statistics from the Centers for Disease Control and Prevention (CDC) reveal that in 2011, 54.6% of high-school girls who consumed alcohol reported binge drinking.

A randomized UK study of menorrhagia shows that the levonorgestrel intrauterine system (LNG-IUS) is more effective than standard medical treatment in reducing the adverse effect of the menstrual problem on women's quality of life.

First know the anatomy and the risk factors, but don't forget to screen for and recognize injury as well.

Certain herbal and complementary medicines may be a valuable treatment option for women with postmenopausal symptoms, according to a new review outlining the advantages and limitations of the available treatments of postmenopausal symptoms.

A prospective U.S. cohort study of nearly 10,000 African-American women published in the American Journal of Obstetrics & Gynecology indicates that child abuse-particularly sexual abuse-is an independent risk factor for uterine leiomyomata (UL).

Researchers in Taiwan have found that women with hypertensive disorders during pregnancy are at a high risk for end-stage renal disease. The risk was much greater for women who had preeclampsia or eclampsia than for those who had only gestational hypertension.

Researchers in Scotland have found that although women whose first pregnancies are complicated by postpartum hemorrhage (PPH) do not have reduced fertility, they do have an increased risk of PPH in later pregnancies. Notably, they also found that women who have cesarean sections at the time of PPH are less likely to conceive again. The study was published January 23 in BJOG: An International Journal of Obstetrics and Gynaecology.

Some may argue that the new ACOG, ACS and other cervical cancer screening guidelines will detect the majority of at-risk women. We should not be content with settling for identifying a majority of at risk women; rather, we should constantly reassess the availability of useful tests to see if we can improve reliability.

Women should wait 12 to 18 months after weight-loss surgery before trying to become pregnant, according to an evidence-based literature review.

Nurse-delivered interventions that combined psychoeducation with supportive attention may help improve mood in patients in whom cancer has been newly diagnosed.

Today, the FDA approved Oxytrol for Women, a patch containing oxybutynin that treats overactive bladder, for over-the-counter sale to women ages 18 and older.

A study published online by the British Medical Journal has shown that women who undergo in vitro fertilization (IVF) are at increased risk of pulmonary embolism (PE) and venous thromboembolism (VTE) during the first trimester.

Analysis of data from 2 longitudinal studies shows a link between early surgical menopause-but not natural menopause-and cognitive decline. The research, supported by the National Institutes of Health, is to be presented at the American Academy of Neurology (AAN) conference in March.

A study published online by The New England Journal of Medicine has determined that vaccination for H1N1 influenza during the pandemic of 2009 did not result in an increase in fetal mortality. The researchers performed an analysis of data on 113,331 women in Norway who became pregnant 43 weeks before December 31, 2010. They used the national health registries and data regarding reimbursement of primary care physicians in Norway to assess the effectiveness of the pandemic vaccine in pregnant women and the effect of vaccination or influenza on fetal survival.

Modifying the surface of pelvic mesh with phosphorylcholine (PC) may help reduce the incidence of mesh-related complications.

Using sophisticated gene sequencing methods, researchers at Yale School of Medicine have demonstrated a regulatory link between stem cell factors that fuel the growth of ovarian cancer and the prognosis of patients, according to a new report.

In high-risk women, a regimen of aspirin cannot prevent preeclampsia but may reduce the incidence of the condition, reported researchers in Finland.

Interventions intended to help pregnant women lose weight may not prevent large-for-gestational-age (LGA) newborns, a study of 2618 women has found.

According to the Centers for Disease Control and Prevention (CDC) Flu View website, the United States is having an early influenza season with most of the country now experiencing high levels of influenza-like-illness (ILI). The CDC defines ILI as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.

A small cross-sectional study suggests that neonates of obese mothers may have lower-than-normal vitamin D levels, even when maternal serum levels are adequate. The findings, published in the Journal of Clinical Endocrinology and Metabolism, underscore the need for more research on the role that vitamin D plays in the health of infants.

This image of gastroschisis on a transverse view of the fetal abdomen was submitted by ObGyn.net reader Viorel Suciu-Lazar, MD. Can you name the numbered structures?

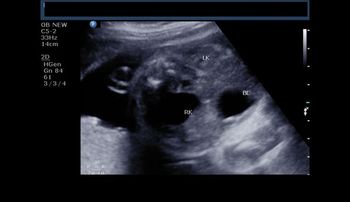
This image of an early pregnancy shows a very obvious pathology. What is your diagnosis?

Iodine declining levels are declining in the United States, and there are potential negative health implications associated with iodine deficiency.

Researchers have found a possible link between sleep-disordered breathing and reduced fetal movements in pregnant women with preeclampsia. An article published in the January 2013 issue of the journal Sleep details the results of a study of patients with preeclampsia who received nasal continuous positive airway pressure (CPAP).

A cross-sectional National Institutes of Health (NIH)-supported study published in Menopauseshows that changes in cognitive function associated with menopause aren’t linear and, in fact, decline is most common in the first year after the final menstrual period.

Researchers in Denmark have tied the use of the antibiotic clarithromycin to an increased risk of miscarriage-but not to an increased risk of birth defects.