
What's coming to Contemporary OB/GYN® this week?
Associate Editor for Contemporary OB/GYN. You can reach her a lcarr@mjhlifesciences.com.

What's coming to Contemporary OB/GYN® this week?

This month, we recognize National Breastfeeding Awareness Month.

A look back at the week's top headlines.

Women with shorter menstrual cycles may reach menopause earlier and experience more severe symptoms overall, according to research in Menopause.

What's in store for the week ahead? Ω

A look back at the week's top stories.

A look back at the week's top stories.

A look back at the week's news.

A look at the cover of this month's issue of Contemporary OB/GYN.

What's coming to Contemporary OB/GYN® this week?

A look back at this week's news.

ObsEva plans to drop linzagolix, its leading candidate for the treatment of uterine fibroids, following concerns raised by the FDA.

Data on COVID-19 during pregnancy, as reported by the CDC, in collaboration with state, local, and territorial health departments and external partners.
What we know about monkeypox in pregnancy, including treatment recommendations, vaccination, and postpartum care.

Mycovia Pharmaceuticals recently announced US availability of oteseconazole capsules for the treatment of recurrent vulvovaginal candidiasis in women without reproductive potential.
Placental cord drainage may be the key to managing the third stage of labor, according to research in BMC Pregnancy and Childbirth.

What's up next in women's health?

The 2022 position statement confirms hormone therapy as the most effective treatment for vasomotor symptoms in menopause and includes updated guidelines for age- and condition-specific treatment plans.

A look back at the week's news.

Contemporary OBGYN® sat down with John Martin, MD, chief medical officer of Butterfly Network Inc., to discuss the Butterfly iQ+, the world's only single-probe, whole-body ultrasound solution.

The CANDLE open-label sub-study enrolled 24 patients with RVVC who failed to respond to fluconazole treatment, which was given as an initial 3-dose treatment over 7 days.

Data on COVID-19 during pregnancy, as reported by the CDC, in collaboration with state, local, and territorial health departments and external partners.

Books and podcasts to help bring self-care and mindfulness into your long weekend:

A look at what's coming to Contemporary OB/GYN® this week.

A look back at the week's news.

Data on COVID-19 during pregnancy, as reported by the CDC, in collaboration with state, local, and territorial health departments and external partners.

The Department of Health and Human Services (HHS) launched a website Friday which offers women a comprehensive list of reproductive health care resources, services, and information.

“The impact was visible and real,” said Xavier Becerra, secretary of the US Department for Health and Human Services during a press conference Tuesday.
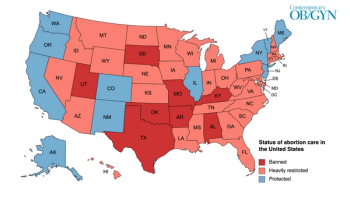
Snapshot of abortion restrictions in the United States.
The US Food & Drug Administration (FDA) sent a response letter to Spero Therapeutics regarding its New Drug Application (NDA) for tebipenem pivoxil hydrobromide (tebipenem HBr) to treat complicated UTIs, saying the application lacks sufficient data.